
Reflecting on President Trump’s first 100 days in office
 Search
Search


 Search
Search

As we have seen in the first article of our series, trademarks play an important role in the protection of your pharmaceutical and life science products and can add significant value. But to understand the benefits but also the limits of your trademarks, in particular with the added complexity of market authorization proceedings for products in the field of pharma and life science, it is of paramount importance to get a sense on the functioning of the trademark system in the EU. So let’s jump right into it!
One of the main goals of the EU is the creation and strengthening of the common market within the EU, and to eliminate any trade barriers between the member states, including with regard to trademarks.
These goals are the basis of the current two-folded trademark system in the EU – national trademarks, on the one hand, and EU trademarks, on the other:
Not only the member states of the EU, but also the EU itself is a member of the so-called Madrid System administered by the International Bureau of the World Intellectual Property Organization (WIPO). The EU can therefore be designated in an International Registration, and an EU trademark can be used as a basis for an International Registration.
Before applying for a trademark, you have to decide on the scope of your protection. There are three main questions each applicant has to sort out before applying for a trademark:
All those information have to be included in the application form, which can then be submitted online or on paper.
Trademark protection has its roots in the idea that the value created by a trademark should not be exploited by competitors or other third-parties. Furthermore, the public should be protected from deceiving signs. However, only trademarks that are used for goods and services on the market are able to create value or any customer expectation. Therefore, the protection is only justified for goods and services where the trademark is or is going to be used. The protection has, thus, to be limited to these goods and services which have accordingly to be specified in the trademark application.
To facilitate the search, filing and examination of trademarks as well as set the costs for a trademark application, the goods and services are classified in a standardized system. For this purpose, the so-called “Nice classification system” is used. This system divides the goods and services into 45 classes (34 for goods and 11 for services). For example, pharmaceutical products are classified in Class 05 and implants in Class 10, whereas medical and pharmacological research services are classified in Class 42 and human healthcare services in Class 44.
Depending on the office, the registration procedure may deviate slightly, but the main stages are the same. For ease of reference, we will therefore concentrate on the procedure for an EU trademark:
The procedure basically can be separated into three stages: (1) the examination period (2) opposition period and (3) registration: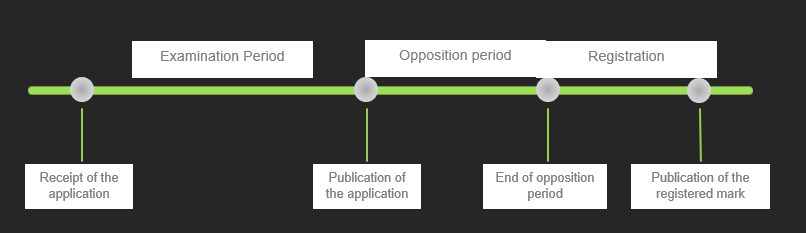
During the examination period, the EUIPO ensures that the trademark application meets all requirements necessary for registration. This includes formal criteria like a correctly identified owner, a clear representation of the mark and a correctly classified list of goods and services, but also substantive criteria such as the fact that there are no conflicting absolute grounds of refusal (e.g. signs that are merely descriptive or non-distinctive). In contrast to some national offices, like Sweden, the EUIPO will not deny the registration based on prior third party rights (so-called “relative grounds for refusal”).
Following the examination procedure, the application is published by the office. The publication sets off the opposition period in which owners of other trademarks or further conflicting IP rights are able to object to the registration of the trademark based on their earlier rights. For EU trademarks, the opposition period is of three months from the date of publication.
If no opposition is filed, the trademark will be registered at the end of the opposition period.
After registration, trademarks are valid for a period of ten years from the date of application and subject to renewal at the request of the proprietor for another ten year period.
After registration, a trademark – in principle – has to be put to genuine use in commerce within the territory for which it claims protection for, e.g. the EU or a respective member state in case of a national trademark. This genuine use has to be in connection with the specific goods and/or services it is registered for. The rationale behind the use-requirement is that – as already mentioned – the protection afforded by a trademark is only justified when a trademark is genuinely used - according to its primary function - as an indicator of the commercial origin of goods and services.
However, the legislature recognizes that it can take time to create and establish a product or service on the market. To give the trademark owner a little “ramp-up-time”, there is an exception to this use-requirement. The trademark owner has a “grace period” of 5 years after registration, during which it is not necessary to demonstrate use of the mark in order to rely upon it.
This is particularly relevant with regard to pharmaceutical trademarks, as it provides a bit of leeway in light of the market authorization process for the pharmaceutical products. Nonetheless, it might happen that the grace period is already expired before the product could be launched on the market due the length of market authorization procedures. The respective consequences and potential remedies will be tackled in a later article of this series.
Trademark protection, although extendable as often as wished by the owner, is finite.
3.Thirdly, a trademark can be declared invalid upon request to the trademark office or in an counterclaim in the course of infringement proceedings. The invalidity action can be based either on absolute grounds (e.g. missing distinctiveness of the trademark or bad faith of the trademark owner at the time of filing the trademark) or relative grounds (i.e. conflicting earlier rights). Except for cases of acquiescence, there is no time limit for the filing of such a cancellation request. Thus, you have to keep in mind that your trademark may, at any point, be attacked by a third party, e.g. based on conflicting prior IP rights.
4. Finally, each trademark owner may surrender its trademark at will in respect of some or all the goods or services for which it was registered.
After giving you an overview of the European trademark system and the functions and registration of trademarks, in our next article we will further discuss trademark registration and focus on the market authorization proceedings.
Authored by Maria Luce and Frederic Urbanek.